Guided Reading 18-2 Radical Revolution and Reaction
Gratuitous Radical Reactions – Chlorination of Methane
Alkanes are pretty boring, chemically speaking. They don't tend to undergo many reactions. However, when they are treated with Cltwo and light, a substitution of H for Cl occurs. Equally we promise to show here, this is Not an acid-base reaction. In fact, it proceeds through a pathway we haven't explored yet, called, "gratuitous radical substitution". In this mail we innovate the concepts of homolytic bond breakage, single-barbed curved arrows, free radicals, and free-radical substitution through the example of chlorination of alkanes with Cl2 and light, which will lay the foundation for this series on free-radical chemistry.
Table of Contents
- Heterolytic Bond Cleavage Versus Homolytic Bond Cleavage
- Chlorination of Marsh gas With Cl2 And Light (hν) Breaks C–H and Forms C–Cl
- Why Chlorination of Methyl hydride With Cltwo Cannot Be An Acid-Base Reaction
- The Reaction Proceeds Through A Neutral Intermediate
- The Chlorine-Chlorine Bond Is Weak. Then How Does It Break?
- "Homolytic" Chlorine-Chlorine Bail Breaking Is Depicted With "Single-Barbed" Curved Arrows
- The Chlorine Cantlet Is Neutral And Has An Unpaired Electron
- Summary: "Complimentary Radicals"Are Highly Reactive Chemic Species Containing An Unpaired Electron
1. Heterolytic Bail Cleavage versus Homolytic Bond Cleavage
With rare exceptions, until now every reaction we've discussed (acid-base, substitution, elimination, addition) has involved the formation of bonds between an electron pair donor (Lewis base) and an electron pair acceptor (Lewis acrid) or the breakage of bonds to generate the same [this is called "heterolytic" cleavage, by the manner, since one bonding partner gets two electrons and the other gets zippo].
In this series of posts we'll take a detour into a corner of organic chemistry where bonds are formed by the combination of single electrons and bonds break through "homolytic" cleavage [that is, each bonding partner receives an equal number of electrons]. As we'll see, these reactions are mostly referred to as free radical reactions.
ii. Chlorination Of Methane With Cl2 And "Low-cal" Breaks C-H and Forms C-Cl
It all starts with a unproblematic ascertainment. Take an ordinary hydrocarbon gas – methane, for example, although any alkane hydrocarbon will be suitable hither. When we combine this hydrocarbon with chlorine gas,in the night, nothing happens.
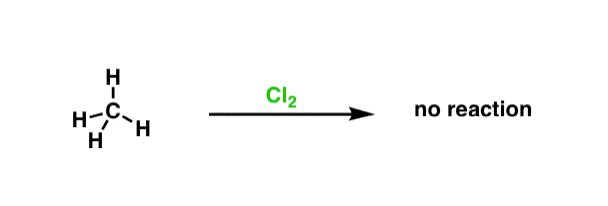
Hither's the interesting function. Moving picture a switch – or remove the embrace – such that visible light tin enter the flask, and suddenly our methane is consumed such that carbon-hydrogen bonds are replaced with carbon-chlorine bonds. The final product depends on the number of equivalents of chlorine gas – let'due south use Cl2 in very small quantities to start with, to go along things simple.
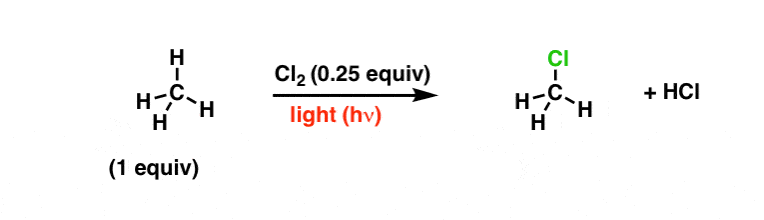
What'due south going on here? Note that "hν" means "light". [We can too do this reaction with rut lone, although it requires higher temperatures].
Before trying to understand why this happened, allow's brand sure nosotros're clear on what has happened.
Let's look at what bonds have formed and what bonds have cleaved. Notice that nosotros're breaking Cl-Cl, C-H, and forming C-Cl.
3. Why Chlorination of Marsh gas With Cltwo Cannot Exist An Acid-Base Reaction
What reactions take we seen so far that would be capable of such a transformation? Well, we've seen that all acid-base of operations reactions involve the cleavage of a bail between H and some cantlet. Then maybe, you might say, information technology's possible that somehow a hydrogen is being pulled off the carbon by a strong base, and then the carbon attacks chlorine. Only does that brand sense here?
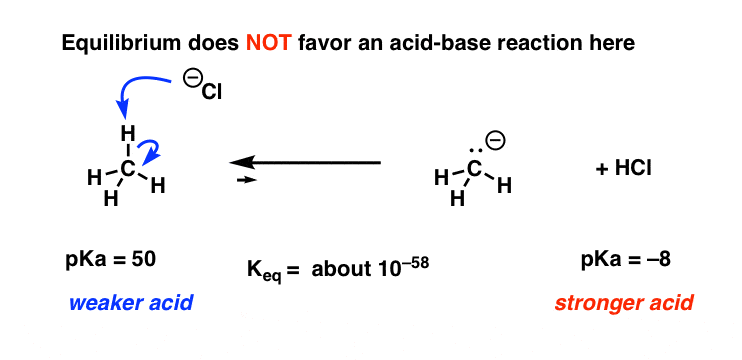
Notation that marsh gas [CH4] would have to be the acid, and chloride ion [Cl- ] would take to be the base. As we've seen earlier, this makes NO sense as an acid base reaction, because we'd be going from a very weak acid [CH4] to a very stiff acid [HCl] and likewise a weak base [Cl] to a strong base.
This is like trying to get Niagara Falls to flow in reverse. Not gonna happen!
4. The Reaction Gain Through A Neutral Intermediate
Furthermore, a second piece of prove should give pause. If the reaction proceeded through some kind of charged intermediate similar Cl- or CHthree – , we would look that the reaction would continue more than quickly in polar solvents [that can stabilize charge] as opposed to nonpolar solvents [which do not stabilize charge]. Instead, we find that the rate of the reaction is about completely independent of solvent polarity. It gain just nigh as quickly in [nonpolar] carbon tetrachloride as it does in a polar solvent such as methanol.
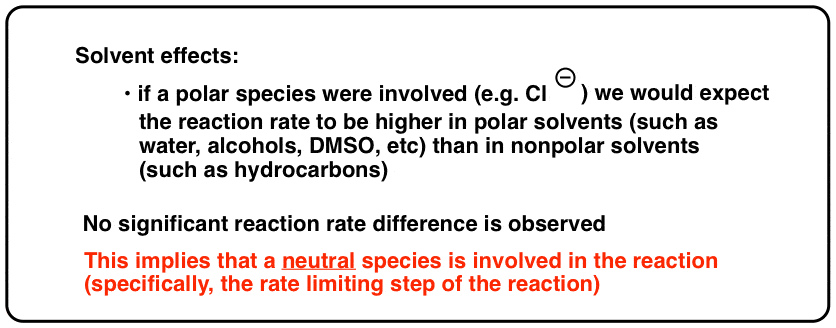
So what does this mean? Information technology is consistent with the reaction proceeding through neutral intermediates rather than polar ones.
Alright – so how, then, might we get a neutral intermediate?
5. The Chlorine-Chlorine Bond Is Weak. Then How Does It Break?
Let's think first near that chlorine-chlorine bond, which is relatively weak. Imagine that by heating it up or past shining light on it [recall that light is a form of energy!] it might break somehow. How might it break?
Choice #1 would wait something similar this – "heterolytic" cleavage, where one atom receives both electrons from the bond, while the other does
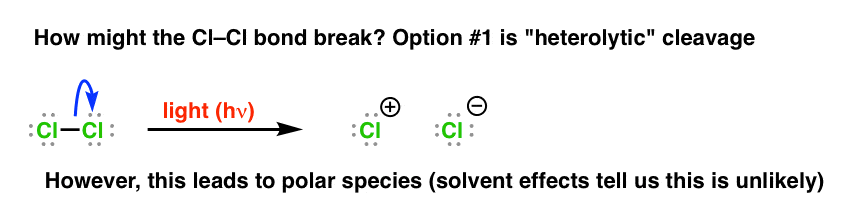
However if this was the case, then we should expect to see a faster reaction in polar solvents, which is non the case.
So what else might happen here?
Recall that many of the molecules nosotros've been discussing take a dipole. That is, two atoms sharing a bail have unequal electronegativities, and thus unequal electron densities – one is electron rich and one is electron poor.
However here we accept two chlorine atoms. They have equal electronegativity. Then we would not expect that one chlorine should "win" the tug of war of electrons over the other.
half-dozen. "Homolytic" Chlorine-Chlorine Bond Breakage Is Depicted With "Unmarried-Barbed" Curved Arrows
So we are left with this: What if the chlorine chlorine bail breaks such as to give each chlorine a single electron?
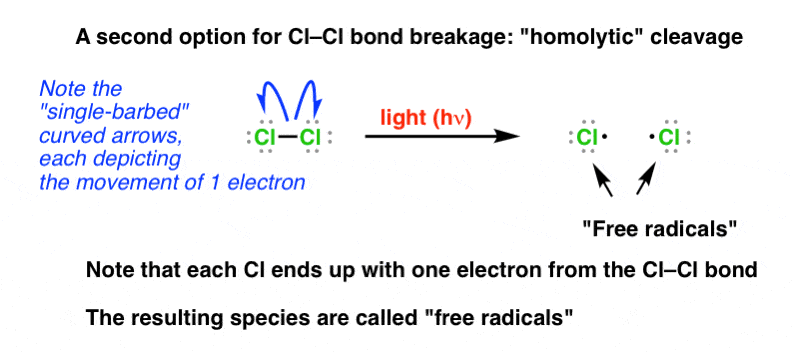
Weird, when you first see it. 1 note – when using the arrow pushing notation, nosotros modify it somewhat, such that we accept single fishhooks showing where the electrons get. Note that we can use these to bear witness a unmarried electron going to each of the 2 chlorines.
But let's wait at this a bit more closely.
7. The Chlorine Atom Is Neutral And Has An Unpaired Electron
What would exist the accuse of the chlorine cantlet? Information technology has 7 valence electrons.
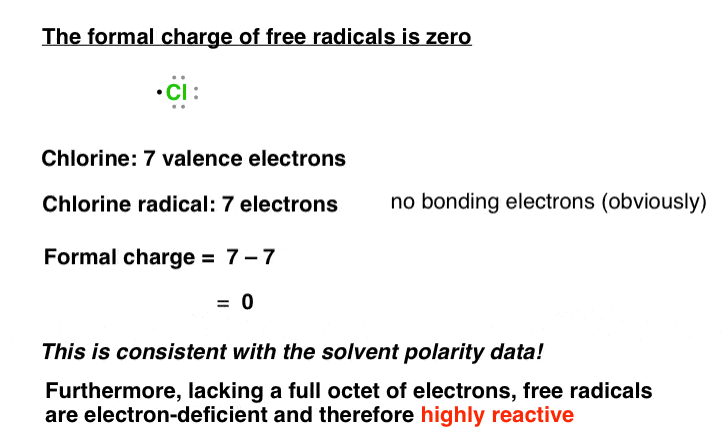
This species is neutral, which is consistent with the solvent data.
Would nosotros expect such a species to be stable? If we recall back to the octet rule, we see that each chlorine atom bears less than a full octet of electrons. In other words, it is electron-scarce.
8. Summary: Species With An Unpaired Electron Are Chosen "Free Radicals" And Are Highly Reactive
Nosotros would expect such a species to exist highly reactive, since there will be a strong driving strength to grade the full octet.
The whole point of this article is to say that yes, such species do exist, and they are chosen "complimentary radicals" [the etymology of this term is interesting, but a little old-fashioned and non crucial for us right now]
There are boosted pieces of evidence collected over the past hundred-odd years to back up the existence of costless radicals. They aren't crucial to empathise, but if you're curious you could endeavor reading well-nigh EPR, CIDNP, or Moses Gomberg, the father of free radical chemical science.
In the next few posts we'll go through the following topics:
- What factors stabilize (or destabilize) complimentary radicals ?
- How do we "know" when a free radical reaction might be occurring?
- What is the mechanism of complimentary radical exchange reactions (such as that above?)
- What useful reactions can we perform using free-radical chemistry?
See yous next postal service!
Adjacent Post: 3 Factors Which Stabilize Gratis Radicals
Source: https://www.masterorganicchemistry.com/2013/07/30/free-radical-reactions/
0 Response to "Guided Reading 18-2 Radical Revolution and Reaction"
Publicar un comentario